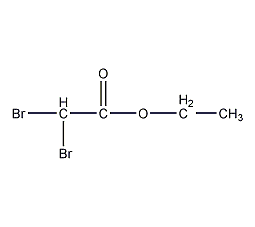
structural formula
| business number | 06hx |
|---|---|
| molecular formula | c4h6br2o2 |
| molecular weight | 245.90 |
| label |
none yet |
numbering system
cas number:617-33-4
mdl number:mfcd00041718
einecs number:210-510-7
rtecs number:none
brn number:none
pubchem id:none
physical property data
1. properties: colorless or light yellow liquid
2. boiling point (℃, 1.0 mmhg): 194
3. boiling point (℃, 12 mmhg): 77
4. density: d 1.9025 g/cm3
5. solubility: insoluble in water, slightly soluble in hexane, ether, methylene chloride, acetone, ethyl acetate, ethanol.
6. relative density (20℃, 4℃): 1.8991
7. refractive index at room temperature (n20): 1.4633
toxicological data
none yet
ecological data
1. other harmful effects: this substance may be harmful to the environment, and special attention should be paid to water bodies.
molecular structure data
1. molar refractive index: 37.77
2. molar volume (cm3/mol): 124.7
3. isotonic specific volume (90.2k ): 317.6
4. surface tension (dyne/cm): 42.0
5. polarizability (10-24cm3): 14.97
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 2.2
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 26.3
7. number of heavy atoms: 8
8. surface charge: 0
9. complexity: 82.1
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
it has tear-inducing effect. avoid inhalation and operate in a fume hood.
storage method
seal the secret container and store it in a sealed main container in a cool, dry place.
synthesis method
it is derived from the interaction between ethyl acetate and bromine in the presence of red phosphorus.
purpose
under the action of potassium tert-butoxide or highly hindered phenoxide, ethyl dibromoacetate and organoborane undergo an alkylation reaction. α-bromo ester or α,α-dialkyl substituted ester (formula 1)[1]. the use of phenoxy groups (pk 11.7) can reduce self-condensation reactions of starting materials or products. this is an alternative to traditional malonate synthesis methods.
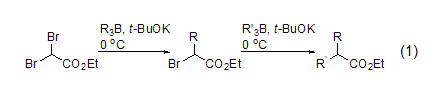
under low temperature conditions, under the action of zinc/silver-graphite, the reformatsky reaction of ethyl dibromoacetate occurs to generate α -bromo-β-hydroxyester (formula 2)[2], then treat α-bromo- with the appropriate group β-hydroxyesters can give a variety of products. zinc/silver-carbon reagents can also be used on aldose [3]. adding diethyl to the organozinc reagent in the reformatsky reaction aluminum chloride can improve the reaction to obtainα,β-unsaturated ester, which is an alternative to the wadsworth-emmons reaction. samarium chloride or chromium chloride it can also be used for this reaction (equation 3)[4].
underthecatalysisoftriethylborane,theadditionreactionofethyldibromoacetatetoketeneacetalyields1,4-diester(formula4)[5].
controlling the reaction conditions, benzenethiol can react with ethyl dibromoacetate to generate the corresponding thioacetal (formula 5)[6]. further alkylation can give pyruvic acid derivatives object.
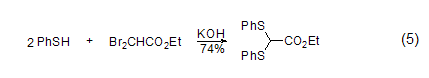
in copper or zinc powder under the action of ethyl dibromoacetate, monoolefins can undergo ethoxycarbonylcyclopropanation reaction with ethyl dibromoacetate (formula 6)[7].
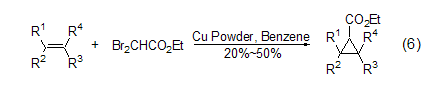

 微信扫一扫打赏
微信扫一扫打赏

