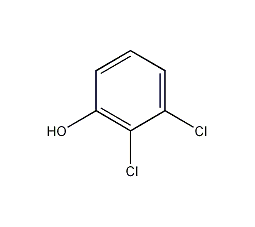
structural formula
| business number | 05v5 |
|---|---|
| molecular formula | c6h4cl2o |
| molecular weight | 163 |
| label |
2,3-dichlorophenol, dichlorophenol, 2,3-dichlorophenol, 2,3-dichloro-pheno, medicine |
numbering system
cas number:576-24-9
mdl number:mfcd00002160
einecs number:209-399-8
rtecs number:sk8450000
brn number:2043615
pubchem id:none
physical property data
1. physical property data
1. appearance: white crystal
2. melting point: 58℃
3. solubility: soluble in ether and chloroform and hot ethanol, difficult to dissolve in water and benzene
4. boiling point (ºc): 206
5. gas phase standard claims heat (enthalpy) (kj·mol-1 ):-168.0
toxicological data
none yet
ecological data
none yet
molecular structure data
5. molecular property data:
1. molar refractive index: 37.92
2. molar volume (cm3/mol): 111.7
3. isotonic specific volume (90.2k): 294.0
4. surface tension (dyne/cm): 47.8
5. polarizability (10-24cm3): 15.03
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 0
5. number of tautomers: 3
6. topological molecule polar surface area 20.2
7. number of heavy atoms: 9
8. surface charge: 0
9. complexity: 97.1
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. properties: white crystal.
storage method
none yet
synthesis method
2. preparation method:
sulfonation of 1,2,3-trichlorobenzene into salt and high-pressure hydrolysis to obtain 3,4-dichloro-
2-hydroxybenzenesulfonic acid is obtained by hydrolysis with sulfuric acid to remove the sulfonic acid group.
purpose
3. usage:
as an organic synthesis intermediate. used in the synthesis of the drug diuretic acid.

 微信扫一扫打赏
微信扫一扫打赏

