[background and overview][1][2][3]
(s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, whose cas number is 118864-75-8, is an important intermediate in the production of solifenacin. its chiral purity and isomeric impurities directly affect the chiral purity and isomeric impurity content of solifenacin, thereby directly affecting the efficacy of solifenacin. the chemical name of solifenacin is 1-azabicyclo[2.2.2]octane-8-yl-(1s)-1-phenyl-3,4-dihydro-1h-isoquinoline-2-methyl acid ester, whose cas number is 242478-37-1, is a selective muscarinic m3 receptor antagonist. it is clinically used to treat overactive bladder with symptoms of urgency and frequency of urination. it is an antispasmodic agent for the urinary system. medicine. (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline chemical formula c15h15n, molecular weight 209.28600, melting point 96-98ºc, density 1.065 g/cm3, boiling point 338.4ºc at 760mmhg, flash point 166.9ºc, refractive index 1.589, vapor pressure 9.87e-05mmhg at 25°c. if inhaled, move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if eye contact occurs, separate the eyelids and rinse with fluid rinse with water or saline and seek medical attention immediately. if ingested, rinse mouth immediately. do not induce vomiting and seek medical attention immediately. advice to protect rescuers is as follows: move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. if there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
【synthesis】[1]
to a solution of 1726 mg of l-tartaric acid (11.5 mmol, 1 equivalent) and 10 ml of methanol, the racemate 1-phenyl-1,2,3,4-tetrahydroisoquinoline (racemate raw material) was added dropwise ) 2750 mg (chemical purity 87.4 mass%, 11.5 mmol) of methanol solution in 10 ml, cooled to 5°c, and crystals precipitated. stir for 1 hour, and then filter under reduced pressure to remove the precipitated (r)-1-phenyl-1,2,3,4-tetrahydroisoquinoline/l-tartaric acid salt crystals (r-type tartrate crystals). the optical purity of (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline s-type raw material in the obtained mother liquor was measured and found to be 83% e.e. 20 ml of water was added thereto, and methanol was distilled off under reduced pressure. to the obtained solution (19.65 g), a 30 mass% sodium hydroxide aqueous solution was added until the ph was 12, and crystals were precipitated. cool to 5°c, stir for 30 minutes, then filter the crystals under reduced pressure, wash with 20 ml of water, and dry under vacuum to obtain (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (s-type raw material) white crystals (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (1092 mg, chemical purity 93 mass%, 4.83 mmol, yield 42 mol%, optical purity 82% e.e.).
[detection][2]
a detection method for (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, using high performance liquid chromatography for detection. the detection steps are as follows:
1) weigh appropriate amounts of (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and (r)-1-phenyl-1,2,3,4-tetrahydroisoquinoline respectively. the two corresponding works of hydroisoquinoline are placed in the same volumetric flask, dissolved and fixed to volume with diluent, and prepared into a system adaptability test solution of a certain concentration; further, the system adaptability test solution is the concentration is 0.8-1.2 mg/ml; further preferably, the concentration of the system adaptability test solution is 1.0 mg/ml.
2) take an appropriate amount of the test product, weigh it accurately, add diluent to dilute it to make a solution containing approximately 1mg per 1ml, and prepare the test solution. it can be understood that the concentration of the test solution is 0.8 ~1.2mg/ml;
3) precisely measure the system adaptability test solution, inject it into the liquid chromatograph, and the injection volume is 15 to 25 μl. further preferably, the injection volume is 20 μl, and record the chromatogram. the number of theoretical plates calculated based on (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline should not be less than 5000, and the separation between isomer peaks should not be less than 1.5. the chromatographic conditions are as follows: chromatographic column: chiralcel od-h; injection volume: 15~25μl; flow rate: 0.8~1.2ml/min; column temperature: 10~40°c; detection wavelength: 228~232nm; mobile phase: n-hexane : isopropyl alcohol: diethylamine, the volume ratio is (480~520): (6~10): 1; diluent: n-hexane: isopropyl alcohol: diethylamine, the volume ratio is (40~60) (40~60): 0.2; detector: uv detector.
[application][3]
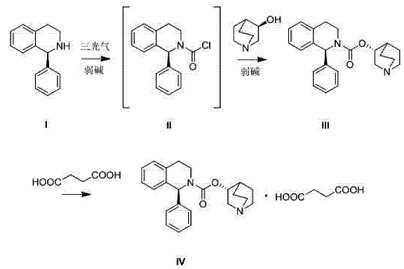
(s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline can be used to prepare solifenacin. solifenacin succinate, cas number 242478-38-2, chemical name 1-azabicyclo[2.2.2]octane-8-yl-(1s)-1-phenyl-3,4 -dihydro-1h-isoquinoline-2-carboxylate succinate is a selective muscarinic m3 receptor antagonist developed by japan’s astellas company. it was first launched in the netherlands, germany, the united kingdom and denmark in 2004. it was launched in china in 2009 and has been sold in more than 50 countries and regions around the world. it has become an important drug for the treatment of overactive bladder (oab) in the european, american and japanese markets, and is recommended by many authoritative institutions and guidelines. it is prepared as follows: using (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (i) in a(s)-1-phenyl-3,4-dihydro-1h-isoquinoline-2-formyl chloride (ii) is obtained by reaction and condensation with triphosgene under alkali (weak base) conditions. compound ii can be separated without separation. , directly heated under the catalysis of an organic base through a one-pot method, and condensation reaction with (r)-3-quinuclidinol to obtain solifenacin (iii), which is then salted with succinic acid to produce solifenacin succinate. new (iv).
[main reference materials]
[1] gengping mori; akira nishiyama. preparation method of (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline. cn200980150249.8, application date 2009-12-14
[2] meng fafa; cao huanyan; xu liang; deng chaoqin; yu weiwen; fan zhiqi. a detection method for (s)-1-phenyl-1,2,3,4-tetrahydroisoquinoline. cn201711085960 .2, application date 2017-11-07
[3] li liwei; yan shaokang; li li; liu e; xu liqun. a synthesis method of solifenacin succinate. cn201410752580.x, application date 2014-12-11

 微信扫一扫打赏
微信扫一扫打赏

