[background and overview][1][2]
2,4-dimethyl-6-tert-butylphenol, chinese alias antioxidant 6bx; 2-tert-butyl-4,6-dimethylphenol; antioxidant la; antioxidant topanol- a; cas1879-09-0, molecular formula c12h18o, molecular weight 178.27100. 2,4-dimethyl-6-tert-butylphenol is an energy the substance that quickly reacts with free radicals to terminate the chain reaction is a new type of high-temperature resistant aviation fuel antioxidant. acrylic inhibitor, also often called a stabilizer, is an intermediate for a variety of polymer antioxidants and is also a very important pharmaceutical intermediates. the alkylation reagents used in the production of tert-butylphenol at home and abroad mainly include isobutylene, tert-butyl alcohol and methyl tert-butyl ether, and the catalysts mainly include acids (sulfuric acid and solid acids, etc.) and resins. 2,4-dimethyl-6-tert-butylphenol is a substance that can quickly react with free radicals to terminate chain reactions. it is also often called a stabilizer. when storing or transporting methyl methacrylate, a certain amount of a substance is added to prevent self-polymerization of methyl methacrylate. if the amount added is too small, it cannot effectively prevent self-polymerization. if the amount added is too much, it will affect future production. therefore, establishing a simple, fast, and accurate method for determining the polymerization content of resistor is of great significance for guiding process operation.
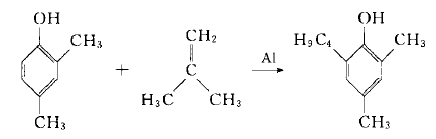
【synthesis】[1]
use 2,4-dimethylphenol and isobutylene as raw materials and concentrated sulfuric acid as the catalyst to perform an alkylation reaction to prepare 2,4-dimethyl-6-tert-butylphenol. the target product purity is 99%, relative to the yield of 2,4-dimethylphenol reaches more than 95%. the specific reaction steps are as follows: in a 100 ml four-neck bottle with stirring, air guide tube and reflux condenser, add 35 g (0.278 mol) of 2,4-dimethylphenol and 0.32 g (3.26 mol) of concentrated sulfuric acid as catalyst under nitrogen protection. mmol), heated to about 90°c, introduced isobutylene for alkylation reaction, and monitored by gc until the raw material disappeared, about 4 hours. after the reaction is completed, lower to room temperature, wash with water until neutral, separate into layers, and dry. the organic layer is distilled under reduced pressure. after gc analysis, the product 2,4-dimethyl-6-tert-butylphenol is obtained with a purity greater than 99%. the yield is more than 95% compared to 2,4-dimethylphenol.
[application]
2,4-dimethyl-6-tert-butylphenol is a substance that can quickly react with free radicals to terminate the chain reaction. it is a new type of high-temperature resistant aviation fuel antioxidant, acrylic polymerization inhibitor, and often it is called a stabilizer and is an intermediate for a variety of polymer antioxidants. it is also a very important pharmaceutical intermediate. examples of its application are as follows:
as an intermediate, it is used to prepare antioxidant 1790 intermediate 2,4-dimethyl-6-tert-butyl-3 chloromethylphenol. this substance is one of the main raw materials for the synthesis of antioxidant 1790. antioxidant 1790 is an important hindered phenolic antioxidant. it has the characteristics of non-coloring and high antioxidant performance. it is an excellent anti-aging additive used in polyurethane, polyolefin, polystyrene, polyamide and other materials. . at present, antioxidant 1790 is mainly produced by the reaction of the aforementioned intermediate and cyanuric acid. as the application of this high-efficiency antioxidant becomes more and more widespread, the demand for research on the synthesis process of itself and the intermediate has also increased accordingly. therefore, studying and optimizing the preparation method of 1790 intermediate has important practical application value. the method is: put 2,4-dimethyl-6-tert-butylphenol, paraformaldehyde, concentrated hydrochloric acid, ionic surfactant and non-ionic surfactant into a reaction vessel, at room temperature and under stirring conditions, hcl gas is continuously introduced until the reaction of 2,4-dimethyl-6-tert-butylphenol is complete; wherein, the molar ratio of 2,4-dimethyl-6-tert-butylphenol to paraformaldehyde is 1: (1.1~1.3); the sum of the dosage of ionic surfactant and nonionic surfactant is 0.2~1% of the weight of 2,4-dimethyl-6-tert-butylphenol; the feeding volume of concentrated hydrochloric acid is 0.4 to 0.6 times the feeding mass of 2,4-dimethyl-6-tert-butylphenol (ml/g).
[main reference materials]
[1] wang haiyang, zhao wei, xu lin, & wang shoukai. (2007). synthesis of 2, 4-dimethyl-6-tert-butylphenol. fine chemical intermediates, 37(6), 26 -27.
[2] su guiyan; wang dongchuan; su jianping. determination of the content of polymerization inhibitor 2, 4-dimethyl-6-tert-butylphenol in methyl methacrylate. chemical industry management, 2017, 23: 206-206.
[3] wang zi; fan xiaopeng; sun chunguang; li haiping; guo pingwu; guan bo; xiong changwu; bi zuopeng. method for preparing antioxidant 1790 intermediate by solvent-free method. cn201711478573.5, application date 2017-12-29.

 微信扫一扫打赏
微信扫一扫打赏

